TECHNICAL PAPER #33
UNDERSTANDING INORGANIC
AND ORGANIC FERTILIZERS
By
Dr. Kenton Brubaker
Technical Reviewers
Dr. Roy L. Donahue
J.W. Fitts
Lee Fryer
VITA
1600 Wilson Boulevard, Suite 500
Arlington, Virginia 22209 USA
Tel: 703/276-1800 . Fax: 703/243-1865
Internet: pr-info@vita.org
Understanding Inorganic and Organic Fertilizers
ISBN: 0-86619-241-7
[C]1985, Volunteers in Technical Assistance
PREFACE
This paper is one of a series published by Volunteers in
Technical
Assistance to provide an introduction to specific
state-of-the-art
technologies of interest to people in developing countries.
The papers are intended to be used as guidelines to help
people choose technologies that are suitable to their
situations.
They are not intended to provide construction or
implementation
details. People are urged to contact VITA or a similar
organization
for further information and technical assistance if they
find that a particular technology seems to meet their needs.
The papers in the series were written, reviewed, and
illustrated
almost entirely by VITA Volunteer technical experts on a
purely
voluntary basis. Some 500 volunteers were involved in the
production
of the first 100 titles issued, contributing approximately
5,000 hours of their time. VITA staff included Maria
Giannuzzi
as editor, Suzanne Brooks handling typesetting and layout,
and
Margaret Crouch as project manager.
The author of this paper, VITA Volunteer Kenton K. Brubaker,
is
Professor of Biology and Director of International Agriculture
at
Eastern Mennonite College, Harrisonburg, Virginia. He
received
his doctorate in horticulture from Ohio State University and
has
had experience in tropical agriculture in Zaire, Bangladesh,
and
Haiti. His current research focuses on the use of organic
fertilizers
in vegetable production. The reviewers of this paper are
also experts in agriculture. Roy Donahue has served as an
agronomist
and forester in Asia, Africa, and South America. J. Walter
Fitts is President of Agro-Services International, Inc., an
agricultural
research, analysis, consultation, and planning firm in
Orange City, Florida. Lee Fryer is President of Earth Foods
Associates in Wheaton, Maryland.
VITA is a private, nonprofit organization that supports
people
working on technical problems in developing countries. VITA
offers
information and assistance aimed at helping individuals and
groups to select and implement technologies appropriate to
their
situations. VITA maintains an international Inquiry Service,
a
specialized documentation center, and a computerized roster
of
volunteer technical consultants; manages long-term field
projects;
and publishes a variety of technical manuals and papers.
UNDERSTANDING INORGANIC AND ORGANIC FERTILIZERS
by VITA Volunteer Kenton K. Brubaker
I. INTRODUCTION
Every farmer and gardener realizes that plants receive some
of
their substance from the soil. Just how much plants depend
on
soil fertility is not always obvious, however, because so
many
other factors also influence plant growth--water, sunlight,
pests, and plant variety (genetics). In regions of the world
where crop yields are extremely high, farmers add large
amounts
of fertilizer, usually in the form of a commercial product,
which
they purchase at considerable expense from a farm supply
dealer.
For example, in the corn belt of the central United States,
yields of over 12 metric tons per hectare (200 bushels per
acre)
may be achieved by using hybrid corn, more than 125
kilograms
(kg) of fertilizer per hectare (100 pounds per acre), and
sometimes
large amounts of irrigation water. Such a farmer may spend
$500 per hectare for fertilizer to produce a crop worth
$1,500
per hectare.
In much of the world such capital-intensive agriculture is
impossible
because of its high cost and often would be unwise due to
the uncertainty of rainfall, insufficient length of growing
season,
or possible lack of demand for the crop at harvest.
Nevertheless,
addition of some fertilizer may be economically justified.
The decision as to whether or not to use fertilizer will
depend on the answers to the following questions:
o
Will fertilizer substantially improve the
yield or
quality of
the crop?
o
Will the increased value of the crop cover
the cost of
the
fertilizer?
o
Are the risks associated with producing the
fertilized
crop (lack of
rain, short growing seasons, pest damage,
unstable
market) low enough to justify the investment
in
fertilizers?
If the answers to all of the above seem to be
"yes," then an
additional set of questions should be asked:
o
What type of fertilizer is needed, and how
much?
o
When and how should it be applied?
o
Will the addition of fertilizer change plant
growth in
such a way that other problems may
develop, like increased
susceptibility to drought or pests, collapse of
the plants
due to stem weakness (called lodging in
grain crops),
or an undesirable change in quality such
as taste,
texture, or nutritional value?
Answers to these questions may not be easy to obtain since
experience
is often essential. Usually the farmer or gardener needs
to experiment with fertilizer use in the field in order to
learn
the advantages or disadvantages. However, fertilizer
experiments
are often very difficult to interpret due to the many crop
growth
variables, so that information about experiments by local
agricultural
research stations may be highly desirable.
II. BASIC SOIL FERTILITY THEORY
LAW OF THE MINIMUM
Crop growth and yield depend on a complex set of growth
factors.
The law of the minimum states that growth or yield is no
higher
than the factor that is most limiting to growth. Some
factors,
such as lack of water or obvious pest damage, are usually
easy
for the farmer to recognize. However, some limiting factors
are
not as easily detected, like the lack of an essential soil
mineral element (e.g., nitrogen, phosphorus, or potassium),
or
the lack of good root growth due to poor soil drainage, or
an
insect or nematode eating the roots. Weed growth or soil
erosion
are other factors that may not be obvious to the grower and
yet
are most likely to limit yield.
The law of the minimum may also be applied to the
restriction of
growth due to the lack of just one soil mineral among the
many
that are essential. If we consider just three of the soil
minerals--nitrogen, phosphorus, and potassium--and assume
that
all other growth factors are adequate, the one mineral that
is
not available in sufficient amount will be the one that
limits
the yield. Figure 1 illustrates the effect of three
different
fig1pg3.gif (600x600)

soil nitrogen levels on yield.
FACTORS LIMITING CROP GROWTH
The first step in considering matters of soil fertility is
to
determine what factor or factors are most likely to limit
crop
growth and yield. For example, if lack of soil fertility is
indicated, then one must find out which nutrient is lacking.
Throughout the world, this element is most often nitrogen.
Several factors can limit plant growth:
o
Lack of water
o
Lack of sunshine
-
growing season too short
-
days too short
-
too cloudy, or crops shaded by trees
o
Lack of oxygen for roots
-
soil water-logged, poor drainage
-
soil too compact, tight
o
Soil too cold; may fail to warm up because
of poor
drainage
o
Competition with weeds or other plants (too
many
plants)
o
Pests and diseases that attack leaves,
stems, fruits,
or roots
-
insects (e.g., beetles, grasshoppers,
aphids)
-
diseases (e.g., wilt, mosaic, blights,
pythium)
-
nematodes
-
birds, rodents, and other animals
o
Lack of soil nutrients due to
-
soil erosion with loss of most fertile
layer
-
soil chemistry, especially improper soil
pH(*)
-
leaching (removal of nutrients by the
movement
of water
downward in the soil) or cropping removal)
o
Crop variety, genetics
--------------------------
(*) pH indicates the acidity or alkalinity of the soil, and
is based
on a scale of about 4.0 to 6.5 (acid), 6.5 to 7.5 (neutral)
and
above 7.5 (alkaline), with the midpoint of 7 indicating the
exact
neutral soil condition. Most plants prefer a pH of about
6.5,
which is slightly acid.
THE NATURAL CYCLE OF PLANT NUTRIENTS: THE NITROGEN CYCLE
Plant nutrients are neither created nor destroyed; they
simply
change their chemical form and move from place to place. The
movement of nitrogen is interesting, complex, and usually
the
most crucial to plant growth, so we will deal with it in
some
detail in this paper.
The earth's atmosphere is the greatest reservoir of
nitrogen; 78
percent of air is made up of this valuable element. Here it
is
present as a pure element, [N.sub.2], a form that most
plants cannot
use. The most important occurrence in plant nutrition is the
process in which the elemental nitrogen of the air is
converted
into forms of nitrogen that most plants can absorb through
their
root systems. This process is called nitrogen fixation.
There are three ways nitrogen from the atmosphere can be
obtained
for use by plants (see Figure 2):
fig2pg6.gif (600x600)
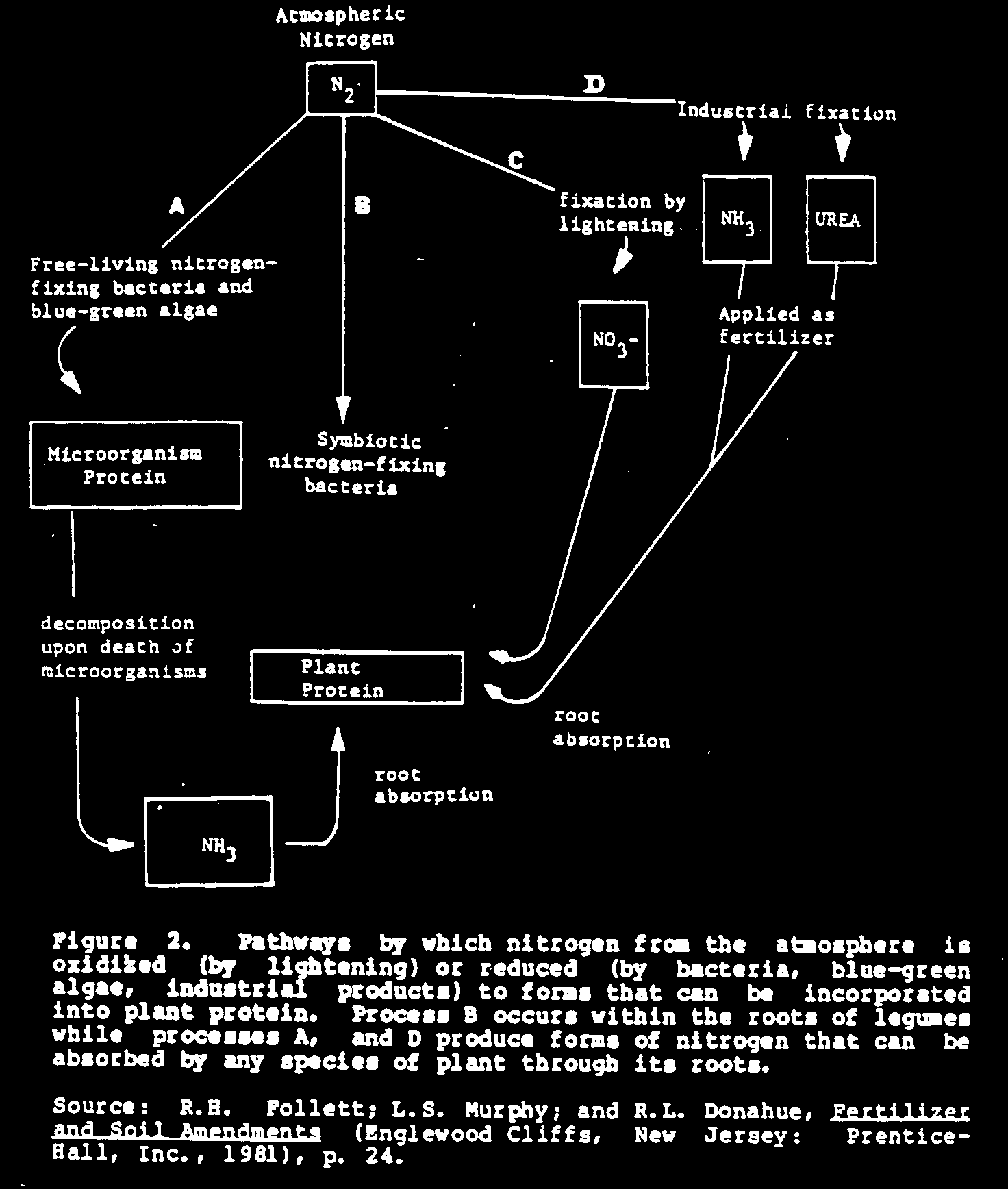
o
capture of nitrogen by nitrogen-fixing
bacteria or
blue-green
algae (a natural process);
o
fixation of nitrogen by lightning in
electrical storms
(a natural
process); and
o
industrial fixation of nitrogen in
fertilizer factories
(an
industrial process).
Nitrogen Fixation by Bacteria and Blue-green Algae
Certain bacteria and blue-green algae are naturally equipped
to
absorb inorganic, elemental nitrogen from the air and
chemically
change it through the addition of hydrogen (called chemical
reduction) to the kind of nitrogen found in the organic
molecules
of plants and animals called protein. The nitrogen of
protein is
present as amine nitrogen, symbolized chemically as the
amine
group, -[NH.sub.2].
By maintaining a well-drained but moist soil, the
free-living,
nitrogen-fixing microorganisms can be cultivated, providing
a
cost.-free source of organic nitrogen. However, these
bacteria
must have an energy source on which to feed, such as straw
or
other plant residue, and this usually limits the amount of
nitrogen
they fix.
Other nitrogen-fixing bacteria live in specialized plant
root
tissues called nodules where they fix nitrogen and make it
available
to the host plant. Plants that contain nodules are usually
legumes, which include members of the bean and pea family. A
nodule that is active in fixing nitrogen will have a pink
color
if it is broken open and examined. The bacteria that live in
nodules are called symbiotic because they benefit their host
as
well as get benefits from the host plant.
The water fern, Azolea, widely used in paddy rice culture,
also
has nitrogen-fixing microorganisms living in its tissues.
These
organisms make nitrogen available to both their natural
host, the
water fern, and to the rice plant. Thus, a farmer or
gardener
who grows legumes or other plants such as Azolea, which have
nitrogen-fixing microorganisms associated with them, is able
to
convert free elemental nitrogen of the air into organic
nitrogen
of the crop plant.
Nitrogen Fixation by Lightning
Another natural process that converts elemental, atmospheric
nitrogen into a form useful to plants is the electrical
discharge,
lightning, which occurs in thunderstorms. This process
oxidizes nitrogen (combines nitrogen and oxygen) forming an
inorganic
nitrogen compound called nitrate ([NO.sub.3]-). This very
water-soluble
fertilizer is readily absorbed through the roots of
plants. Electrical storms may contribute a substantial
amount of
nitrogen to the soil in some areas, although the heavy
rainfall
associated with such storms may tend to wash the nitrate out
of
the plant root zone fairly quickly. For this reason, a well
developed root system, such as that of trees and grasses, is
essential to capture this form of naturally-fixed nitrogen.
Industrial Nitrogen Fixation
A third process of fixing atmospheric nitrogen is
accomplished by
modern chemical technology in industrial facilities. This
process
uses natural gas and other hydrocarbon fuels to produce
ammonia ([NH.sub.3]), ammonium ([NH.sub.4]+), and urea
([NH.sub.2] Q/[CNH.sub.2]), both useful
forms of chemically reduced nitrogen. Ammonia can be
considered
inorganic nitrogen, while urea is an organic form of
nitrogen
because it contains carbon.
Table 1 summarizes the forms of nitrogen obtained from the
earth's atmosphere.
SOME SOURCES OF NATURAL NITROGEN FERTILIZER
A rich and valuable natural source of nitrogen fertilizer is
the
oxidized, ancient deposits of bird and bat manure, known as
guano, which occur in various locations around the world,
especially
in coastal regions and caves. The nitrogen in guano,
which is collected and sold as fertilizer, is usually
combined
Table 1.
Forms of Nitrogen Obtained from the Atmosphere
Forms of
Chemical
Nitrogen
Formula
Comments
Atmospheric nitrogen
[N.sub.2] Not
available to plants except
certain bacteria and
blue-green
algae.
Protein or amine
-[NH.sub.2] Organic
nitrogen produced by
nitrogen
nitrogen-fixing bacteria and
blue-green algae and
incorporated
into the proteins of
the
microorganisms or the
host
plant when the
microorganism
is symbiotically
associated
with the host plant.
Nitrate nitrogen
[NO.sub.3]- Inorganic
nitrogen produced
when lightning
oxidizes
atmospheric nitrogen.
Ammonium
[NH.sub.4]+ Inorganic
nitrogen produced by
industrial fixation
of
atmospheric nitrogen.
Urea
[NH.sub.2]-O/C-[NH.sub.2]
Organic nitrogen produced by
industrial fixation
of
nitrogen and hydrogen
from
natural gas, coal, or
oil.
with potassium (K) or sodium (Na), forming potassium nitrate
([KNO.sub.3]) or sodium nitrate ([NaNO.sub.3]).
Another important natural source of nitrogen fertilizer is
fresh
or composted animal manure and human wastes. These are a
complex
mixture of several forms of nitrogen including urea
(organic),
protein (organic, mostly bodies of microorganisms), nitrates
([NO.sub.3]), ammonia ([NH.sub.3]), and, ammonium
([NH.sub.4]+) compounds. The value
of animal and human manures as fertilizer depends on how the
manure is handled, since it is a rich culture of bacteria,
both
living and dead, and various forms of nitrogen. If the
manure is
exposed to oxygen, the reduced forms of nitrogen (protein,
ammonia,
and urea) may be changed to nitrate by bacteria, or the
population of bacteria may increase dramatically and
incorporate
most of the nitrogen as protein in their own cells. If the
manure is handled so as to exclude oxygen (kept wet or
tightly
packed to exclude air), bacteria growth may be limited and
the
nitrogen will be mainly kept in the reduced forms (ammonia,
ammonium, urea, and protein).
Whether or not the manure is kept under shelter to protect
it
from rain is also crucial since urea and nitrate nitrogen
are
easily washed out of the manure. Ammonia nitrogen is also
readily lost to the air as it is quite volatile, but in the
soil
it changes to ammonium ([NH.sub.4]+) and is absorbed by
clay.
Since the nitrogen content of animal manures is so easily
lost,
several management suggestions should be followed:
o
Keep the manure under a roof to prevent
leaching of
nutrients
that dissolve easily in water.
o
Incorporate it into the garden or field as
soon as
possible to
prevent loss of ammonia (or ammonium).
o
Use a cement floor for storage to prevent
loss of the
liquid
portion in which most of the urea and nitrate is
found.
Sufficient bedding to absorb the urine also
saves urea.
o
Compost human manures thoroughly to ensure
that
diseases and
parasites are killed. (A description of
appropriate
methods of composting human wastes is beyond
the scope of
this paper.
Another source of nitrogen fertilizer is compost, a
decomposing
mixture of plant materials and manure. The nitrogen content
of
compost is usually very low unless it contains substantial
amounts of legumes and manure and is handled with the same
care
as manure. The state of decomposition would also influence
the
percentage of available nitrogen it contains.
A final natural source of nitrogen fertilizer is the use of
crops, especially legumes, as green manure. Crops that are
naturally
high in nitrogen are turned under and allowed to decay, thus
releasing the nitrogen they obtained from the air through
the
activity of the symbiotic bacteria in their nodules.
Decomposition microorganisms play an important role in the
natural cycle of nitrogen. Nitrogen may be lost from the
plant-animal-soil
phases of the cycle when certain soil microorganisms
convert nitrates into elemental nitrogen, which then escapes
back
into the atmosphere. This loss seems to occur most readily
when
the soil is water-logged and microorganisms are forced to
turn to
nitrates ([NO.sub.3], [NO.sub.2], and NO) for their source
of oxygen. Naturally,
this loss of valuable fertilizer nutrients should be avoided
if at all possible by seeing that the soil is well drained
and
thus well supplied with oxygen from the atmosphere. A well
drained soil that permits good oxygen entrance can be
produced
by good cultural practices, especially by the addition of
organic
matter.
To sum up, then, management of the nitrogen cycle may be the
most
important activity a farmer carries out in relation to soil
fertility. The lack of usable nitrogen is the most frequent
cause
of poor crop growth and yield in most soils around the
world.
The nitrogen of the atmosphere is made available to plants
only
through nitrogen-fixation. The growth of both free-living
and
symbiotic bacteria can be managed to increase the amount of
nitrogen in the plant growth cycle. Both symbiotic and
free-living
microorganisms grow well in moist, well-aerated soil.
The chemical state of nitrogen must be appreciated to manage
the
cycle successfully. Organic nitrogen in mainly protein, and
the
important waste product, urea. Such nitrogen is said to be
chemically reduced or combined with hydrogen. Upon
decomposition
of protein and urea by bacteria, the nitrogen is released as
a
volatile gas, ammonia. This reduced form of nitrogen can be
absorbed by plant roots, and it can also be converted by
bacteria
to an oxidized, non-volatile form, nitrate, which is also
readily
soluble and absorbed by plant roots.
Commercial fertilizers may be in the form of ammonia,
ammonium
salts, urea, or nitrate, all of which can be quickly
utilized by
plants. Urea quickly changes to ammonium and can then be
absorbed by plants. Green manures and the protein components
of
animal manures must be changed to ammonium and nitrate
before
they can be absorbed by plants. Before conversion to soluble
forms of inorganic nitrogen, the insoluble organic nitrogen
of
green and animal manures forms a reservoir Of nitrogen that
will
be released slowly (through bacterial decay) during crop
growth.
This slow release prevents its rapid loss during heavy
rainfall.
Highly soluble fertilizers like urea and nitrate are quickly
lost
when leaching occurs. Ammonia can also be lost as a gas, and
nitrate can be changed to elemental nitrogen by
oxygen-starved
soil microorganisms and lost to the atmosphere.
INORGANIC AND ORGANIC FERTILIZERS
Inorganic fertilizers are generally salts of metals such as
sodium, potassium, calcium, and magnesium. Ammonia can also
act
as a carrier of other inorganic nutrients when it occurs in
the
form of a salt of ammonia (ammonium salt). Several important
inorganic fertilizer salts are listed in Table 2.
Table
2. Some Important Inorganic Fertilizer Salts
Name of
Chemical
Percentage
Fertilizer Salt
Formula
of Nutrient
(Elemental)
Ammonium nitrate
[NH.sub.4] [NO.sub.3]
33.5% nitrogen
Di-ammonium
[([NH.sub.4]).sub.2][HPO.sub.4]
-21%, nitrogen
phosphate
23% phosphorus
Superphosphate Ca
[(H.sub.2][PO.sub.4]).sub.2].[H.sub.2]O
20% phosphorus
Dolomite
Mg[CO.sub.3] Ca[([CO.sub.3].sub.2]
10-20% magnesium
Source: N. Brady, The Nature and Properties of Soil (New
York,
New York: MacMillan and Sons Publishing Co., 1984).
Note that each of these fertilizer salts contains a certain
percentage
of the nutrient element based on the relative weights of
all the atoms in the molecule.
Chemically speaking, organic molecules, and thus organic
fertilizers,
are those that contain carbon in organic form. The organic
molecules we have considered so far are protein and urea.
Living
organisms contain many other important organic molecules
including
carbohydrates and nucleic acids. Any fertilizer whose
nutrients
are present mainly in organic molecules like urea, protein,
or nucleic acids is called organic fertilizer. In general,
such fertilizers (compost, manure, and cottonseed meal) have
a
low nutrient content and release these nutrients very
slowly.
This is because bacteria and fungi must first decompose the
organic molecule for the nitrogen to be freed as ammonia or
the
phosphorus to be released as phosphate. Urea is an important
exception to this general rule; it has a very high nitrogen
content (46 percent) and is readily available for plant root
absorption after a day or two when it has been converted by
bacteria to ammonium salts.
Some examples of organic fertilizers with approximations of
their
nutrient content are given in Table 3.
The highly variable nutrient content of organic fertilizers
makes
their use more complicated than that of inorganic
fertilizers,
especially if the grower intends to achieve very high
yields.
This is because the content and form of nutrients is
unknown, or
only approximately known. Also, the generally low nutrient
content
of the organic fertilizer makes it necessary to add very
large
quantities of the fertilizer to the soil. The third
complicating
factor in the use of organic sources of nutrients is the
slow
release of most of the organic nitrogen and phosphorus. The
organic matter must first be decomposed by soil
microorganisms,
which in turn must also die and decompose, before a
substantial
amount of these nutrients is available to plant roots. For
example,
suppose that the organic fertilizer to be used is compost,
green manure, or animal manure--or a combination of any of
these.
If the approximate analysis of the organic material is
0.5-0.1-0.3
(nitrogen-phosphorus-potassium), how much would be needed
per
hectare to furnish the nutrients to produce 6 metric tons of
corn
(100' bushels per acre)?
One estimate suggests that the following amounts of
available
nutrients are needed to produce such a yield.
Nitrogen
Phosphorus
Potassium
(Kilograms)
(Kilograms)
(Kilograms)
Total needed to produce six
metric tons of corn/hectare
168
67 134
Table
3. Total Nutrient Content Of Some
Organic Fertilizers
Total
Nutrient
Content
(Approximate
Percentage)
Fertilizer
Nitrogen
Phosphorus
Potassium
O
Urea ([NH.sub.2] [CNH.sub.2])
46
0 0
Guano (bat or bird fecal
10
2
2
deposits)
Compost (highly variable)
0.1-0.3
<0.1 0.1-0.3
Green manure (legumes)
0.2-0.5
<0.1 0.2-0.4
Horse, cow, or hog manure
0.7
<0.l 0.5
Poultry manure
1.0
0.3
0.3
Sewage sludge
2-6
1-2
0.1-0.4
Dried fish scraps
6-10
2-4 --
Cottonseed meal
6-9 1-2
1-2
Bone meal
2-3
10-15
--
Wood ashes
--
0-1
2-6
Source: Florida
Cooperative Extension Service, Organic Vegetable
Gardening, Circular 375-A (Gainesville, Florida: University
of Florida, Institute of Food and Agricultural Sciences, May
1973).
If we added 50 metric tons of organic fertilizer per
hectare, the
following amounts of nutrients would be supplied:
250 kg nitrogen,
50 kg phosphorus; and 150 kg potassium
However, only about 30-50 percent of the nitrogen and
phosphorus
would be available the first growing season due to the slow
process
of decomposition of the organic matter.
About 50 percent or
more of the potassium would be available.
In conclusion, it becomes
obvious that supplying all nutrients in organic form is a
rather uncertain and labor-intensive practice.
As a result, organic
fertilizers may need to be supplemented with chemical
fertilizers.
Application of 50 metric tons of organic matter to a hectare
(500
kilograms/are(*)) is a huge job.
Furthermore, availability of that
much material may also be a problem, and working the organic
matter into the soil may require a large expenditure of
energy.
Addition of large amounts of organic matter to the soil may
also
lead to a phenomenon known as "nitrate
depression," where the
soluble nitrogen gets incorporated in the bodies of soil
decomposers
until the carbon of the organic matter is decomposed.
For
this reason, the straw (cellulose) of organic matter should
be
decomposed rather thoroughly before it is used as
fertilizer.
Adding nutrients to the soil in the form of organic matter
is not
easy, but it can be done.
The process is an imitation of the
natural fertility cycle of a forest, grassland, or
pond. Experience
and wise management plus a lot of hard work are essential
to making the process work successfully.
Alternative methods of adding large amounts of organic
matter
should be evaluated.
Composting is essential to decrease the
carbon content of the plant material that is added to the
compost
heap, thus permitting more rapid release of the nitrogen and
phosphorus when the material is added to the soil.
Another
important technique is to use the partially decomposed
organic
matter as a mulch, thus allowing the composting process to
continue
on the surface of the ground.
The mulch that remains on
the soil surface at the end of the growing season may then
be
incorporated into the soil as compost.
A third alternative is to
incorporate fresh or partially composted organic matter into
the
soil just before a fallow period, allowing soil
microorganisms to
begin decomposition during a winter or dry season period
when
crops are not growing.
Little soil microorganism activity occurs
during such a fallow period, but some beneficial
decomposition does take place.
------------------------
(*) One are = 100 square meters = .01 hectare.
COMMERCIAL FERTILIZER FORMULATION
Suppose we wanted to make a complete inorganic fertilizer,
that
is, one containing nitrogen, phosphorus, and potassium, all
derived
from inorganic fertilizer salts.
If we mixed potassium
nitrate and ammonium phosphate, we would have such a
fertilizer.
To give a simple example, suppose we mixed 100 kilograms of
potassium nitrate ([KNO.sub.3]) and 150 kilograms of
ammonium phosphate
[([NH.sub.4]).sub.2] [HPO.sub.4] to make 250 kilograms of
complete fertilizer. Let
us calculate how much of each element would be present in
this
batch of fertilizer.
Nitrogen
Phosphorus Potassium
(Kilograms) (Kilograms)
(Kilograms)
100 kilograms KNO
(14%N, 39%K)
14
0
39
150 kilograms (NH) HPO
(21%N, 23%P)
31.5
34.5
0
250 kilograms
45.5
34.5 39
We can now calculate the percentage of each element
(analysis) in
this mixed fertilizer as:
Nitrogen =
45.5 kg/250 kg = 18 percent
Phosphorus =
34.5 kg/250 kg = 14 percent
Potassium =
39.0 kg/250 kg = 16 percent
We would label this an 18-14-16 fertilizer.
In commercial trade,
this would be considered a high-analysis fertilizer because
it
contains a fairly high content of nutrients and no filler.
Many commercial fertilizers, at least those that are
relatively
inexpensive, have a lower analysis, like 5-10-10.
In such a
fertilizer, the inert material (filler such as sand or
sawdust)
would be 75 percent of the weight.
If one needed to transport
the fertilizer a long distance, this non-nutrient weight
should be considered.
High-analysis fertilizers give more nutrients
per kilogram but they often require special care in
handling and storage.
For example, they must be kept dry because
the salts readily pick up water and so are packaged in
plastic-lined
bags and stored in dry areas.
Anhydrous ammonia, a very
high-analysis nitrogen fertilizer, is handled as a liquid
under
pressure in corrosion-resistant tanks.
Many dry fertilizers are
granulated and coated with clay and wax to make them easier
to
store and handle.
The coating may also slow the release of the
nutrients when added to the soil; this slower release may be
desirable. Moreover,
the inert material may contain some trace
elements that may be absent in high-analysis fertilizers.
DETERMINING THE NEED FOR FERTILIZERS
Observation of Visual Symptoms
Under severe deficiency conditions, a trained plant
nutritionist
can diagnose the need for a particular fertilizer element by
examining the growth of the affected plants and the plants'
symptoms. For
example, nitrogen-deficient plants are small and
have a yellowish appearance, especially the lower leaves.
Potassium-deficient plants may show dead tissue around the
edges
of lower leaves and other symptoms such as missing kernels
in
ears of corn.
Iron-deficient plants usually show a marked yellow
color (chlorosis) at the growing tips of the plant.
However, the
use of visual symptoms is not a reliable method of assessing
the
need for fertilizers.
Many factors limiting plant growth (e.g.,
nematode damage or magnesium deficiency) will cause similar
plant symptoms.
Also, when several factors are involved, the
visual symptoms can become very confusing.
Even experts have
difficulty identifying a deficiency by visual observations.
Moreover, by the time visual symptoms occur, so much damage
has
already taken place that correction of the problem is too
late to
be of much value for the current crop.
Soil and Tissue Testing
Analyzing the soil before planting and testing appropriate
tissues
before visual symptoms occur are better methods of
determining
the need for fertilizers.
Soil or tissue samples are
usually sent to a central laboratory, which then gives
advice on
fertilizer needs.
Portable kits are also available to test soil
and tissues but require a good understanding of their use
and
limitations. In
general, portable soil-testing kits are used best
in conjunction with a standard soil and tissue testing
laboratory.
Experimental Testing and Crop Yield
The best method of assessing the need for fertilizer is
actual
field trials in which various combinations of plant
nutrients are
applied to the soils and crops in question.
Again, this procedure
needs to be carried out with great attention to experimental
design but finally becomes the basis for other techniques
such as
soil analysis. Such
field trials are usually carried out by research
centers. In most
developing countries, a farmer or gardener
can often determine the need for fertilizer by fertilizing
only a part of a field or garden and observing the results.
III. ALTERNATIVE
SYSTEMS OF CROP FERTILIZATION
NATURAL SYSTEMS USING SOIL-ENRICHING FALLOW
All successful crop production systems that do not rely on
the addition of fertilizers must imitate the natural cycle
that
existed in the region before the land was cultivated and
devoted
to raising crops.
This principle is most clearly seen in the
"slash-and-burn" or "swidden" agricultural
method of the tropics.
With this practice, a forested area that appears to be
suitable
for cropping is first selected for clearing.
The forest
demonstrates its fertility by the vigor of plant growth,
both
trees and undergrowth.
The farmer can possibly evaluate the
yield potential by feeling, smelling, and tasting the soil,
and
by observing forest growth.
A fertile soil feels soft and crumbly,
smells somewhat like new-mown hay, and tastes slightly sour.
In the tropics, larger amounts of plant nutrients are stored
in
the existing vegetation than in the soil.
With the "slash-and-burn"
practice, this reservoir of plant nutrients is returned to
the soil surface as ash through careful burning of the mass
of
vegetation. Burning
may also help kill pests in the soil including
weed seeds. A
mixture of crops is then planted, including
legumes as well as many other plants whose size and
placement
imitates the forest structure they have replaced.
After two or three years of crop production, the yield
decreases
to the point where weeding no longer seems practical and the
field is allowed, or encouraged, to return to mature forest
as
rapidly as possible.
Many slash-and-burn farmers cherish the
sprouting trees that will regenerate the nutrient stores of
the
mature forest. The
roots of these trees and vines will penetrate
deeply into the soil and retrieve nitrogen and other soluble
nutrients that will have leached from the topsoil during the
brief period of cropping.
This forest fallow (regrowth) may require
12-20 years to regenerate soil fertility.
Certain practices
such as the planting of tree legumes could possibly hasten
this
regeneration, but the cycle cannot be shortened too much or
the
soil will be permanently damaged.
Unfortunately, population
pressures in many areas force farmers to re-use fields
before
they have fully regenerated, and crop yields have declined
accordingly.
Other cropping systems such as wet rice paddies also imitate
the
natural swamp ecosystem, but these may be associated with an
annual flooding cycle, and so are not dependent on a
vegetation
regeneration process.
The flooding brings a substantial quantity
of nutrients from the eroding hillsides farther up the
valley.
Flooding also makes soil nutrients such as phosphorous more
readily available.
CROP ROTATION WITH GREEN MANURES
A system widely practiced before about 1950 in the temperate
agricultural regions is crop rotation.
Here cash crops such as
corn and wheat are rotated with soil building crops such as
clover, alfalfa, or beans, usually soybeans.
Some of the soil-improving
crop may be removed as hay or, for beans, seeds to sell,
but as much as possible is returned to the soil as a way of
building up the nitrogen content of the field.
Before the wide
use of commercial fertilizers, this was one of the most
important
practices of temperate agriculture.
In combination with the use
of manure (the next alternative discussed), it is still
practiced
by a small group of farmers known as "organic"
farmers. These
farmers may also use limited amounts of commercial
fertilizer
(the last alternative described below).
COMBINING CROP PRODUCTION AND ANIMAL HUSBANDRY
Many farmers find that the incorporation of animals into
their
agricultural system is crucial to crop production.
The manure
from these animals is carefully placed on the fields.
Gardeners,
with a smaller area to cultivate, may incorporate animal
manures
into a composting system, thereby increasing the quantity
and
quality of the organic fertilizer they use to fertilize their
gardens. Chinese
farmers have developed especially intricate
systems of using both animal and human manure (known as
night
soil) in the production of crops.
The integration of hogs and
fish into these systems is also crucial to food production
programs.
To make compost, a partially decayed mixture of mostly plant
fig3pg20.gif (600x600)

material, the following points should be kept in mind:
o
Use plant residues as rich in nitrogen as
possible and
supplement
with animal manure. Materials rich in
nitrogen include legumes and animal
materials (e.g.,
fish
scraps).
o
Chop as finely as practical and mix the
materials from
time to
time, if you wish to achieve more rapid decomposition.
o
Keep moist but not saturated so that air
is available.
o
Add superphosphate or rock phosphate to
help prevent
the loss of
ammonia.
o
Add a small amount of already partially
decomposed
compost or
rich-garden soil to promote favorable decomposition.
It will
inoculate the compost with useful
bacteria
and fungi.
o
Keep the compost heap large enough to
ensure uniform
heating but
not so large that air is excluded (a minimum
of about
two square meters). A compost heap that
is
too small
will not heat adequately enough to destroy
weed seeds
and pathogenic organisms.
APPLICATION OF COMMERCIAL FERTILIZER
When it is impossible or impractical to use natural methods
of
maintaining soil fertility, the addition of commercially
produced
fertilizers is necessary.
They can also be used to supplement
any of the above alternatives.
Applying the proper kind and amount of fertilizer is
crucial,
since these materials are highly concentrated and often
expensive.
The kind and amount of fertilizer must usually be determined
experimentally and should be adapted to the soil and
location.
Usually the fertilizer is placed in the soil below and
beside the seed so that the growing roots can quickly begin
to
feed on the nutrients.
Under no circumstances should chemical
fertilizers be mixed with seed; to do so will kill the
germinating
seed. Applications
of fertilizers, especially nitrogen,
may be spaced out over the growing season in regions of very
high
rainfall.
IV. CHOOSING THE
BEST SYSTEM OF CROP FERTILIZATION
ADVANTAGES AND DISADVANTAGES OF THE FOUR ALTERNATIVE SYSTEMS
Natural-Soil Enriching Systems
On the plus side, these systems
o
Are inexpensive because a free service of
nature: forest
growth,
annual flooding, natural reseeding.
o
Provide many benefits in addition to
increasing soil
fertility
that the farmer may not even be aware of,
such as
recycling of trace minerals and pest control
processes.
o
Offer ecological stability and genetic
diversity because
they are
part of a complex natural system with
many plant
species cooperating with one another.
On the other hand, such systems
o
May require years to regenerate fertility,
thus requiring
a
substantial percentage of land in fallow.
Where a
severe
deficiency occurs, such as very low levels of
phosphorous
in the soil and soil-forminq materials,
natural
soil-enriching systems do not replenish these
elements.
o
Are difficult to manage if poor or
undesirable tree or
weed growth
occurs.
o
Are not easily adapted to mechanized crop
production;
thus,
natural soil-enriching systems are labor intensive.
o
Will not support large populations.
Crop Rotation with Green Manures
The advantages of crop rotation with green manures include:
o
Free source of nitrogen through
nitrogen-fixation,
where legumes are grown in the
rotation.
o
Green manure crops control soil erosion
and may control
some weeds.
o
Green manure crops not only improve soil
fertility but
also
dramatically improve soil structure and increase
organic
matter content.
o
May be combined with animal production.
Some of the disadvantages include the following:
o
A considerable amount of land must be used
for green
manure,
taking it out of production.
o
Incorporating the green manure crop into
the soil may
require
considerable animal or mechanical power to turn
the soil.
o
The cost of good seed may be prohibitive.
o
Inoculation with suitable bacteria may be
essential.
o
Green manure crops often deplete soil
moisture, leaving
a dry soil
for the succeeding crop.
Integration of Crop Production and Animal Husbandry
Integrated systems have a number of advantages.
These include:
o
Animals provide valuable manure; they can
also graze on
land
unsuitable for cultivation and eat roughage unsuited
for human
consumption, turning these materials
into manure
and animal products.
o
Animals can help diversify the range of
agricultural
products
and give work when crops do not require attention.
For
example, fences can be repaired and manure
handled at
times when work in the crop fields is not
necessary.
o
Draft animals help work the land and carry
products to
market. Cattle may also be
driven to market for sale.
o
Animal products (meat, milk, cheese, eggs)
improve the
nutritional
quality of the human diet.
o
Animal manure will improve the composting
process,
furnishing
nitrogen for microorganism growth and ensuring
better
completion of the decomposition process.
o
Like green manures, animal manures also
greatly improve
soil
structure.
On the other hand,
o
Animals may be expensive and require
special skills and
resources
not readily available, such as veterinary
services
and high protein feed supplements.
o
Animals require that a certain amount of
land be devoted
to pasture
or other animal feeds; this land must
be fenced
to protect crops.
o
Animals require constant care, which may
be difficult
to provide
during busy crop production periods.
o
Animal manure may be a source of
distributing weed
seed,
insects, and some disease organisms.
Application of Commercial Fertilizers
Some of the advantages of the use of commercial fertilizers
are:
o
A fertility program can be designed
especially for a
particular
crop under specific soil conditions.
o
By selecting the proper fertilizer, rapid
or slow release
of the
nutrient can be regulated.
o
High yielding plant varieties can be used,
especially
the so
called "miracle hybrids."
These new hybrid
varieties
are designed to produce higher yields in
response to
additional fertilizer and water. Their
genetic
potential has been increased through plant
breeding
techniques.
o
Land that has been depleted of nutrients
can be rapidly
rejuvenated
in many cases.
o
Irrigated lands can be farmed intensively.
o
Large urban populations can be sustained.
As with the other systems, commercial fertilizers have
drawbacks.
These include the following:
o
The cash investment may be prohibitive.
o
Often other supporting technologies are
needed along
with
fertilizers, such as irrigation and pesticides,
further
increasing the cash investment. This
means that
a whole
"package" of technology may be required as
yields are
increased through new programs of fertilization.
o
The fertilizer may be applied incorrectly
(excessive
amounts,
wrong type, incorrect placement, or wrong
time).
o
Commercial fertilizers add only nutrients;
they do not
improve the
soil structure. Unless good soil
structure
is
maintained, the soil will deteriorate, and increasing
amounts of
commercial fertilizers will be required
to maintain
a given level of production.
o
Facilities for handling and proper storage
of the fertilizer
may be
inadequate.
ASSESSMENT OF LOCAL CONDITIONS AND RESOURCES
In choosing a new crop fertilization system, or more likely,
in
modifying a current system, one must realistically assess
local
resources. First, it
is important to analyze carefully the
system currently being used.
It may be useful to concentrate on
the movement of nitrogen through the cycle, and note where
improvements of nitrogen availability to plants can be
achieved.
Perhaps commercial nitrogen fertilizer could be applied on
certain
crops to find out if additional nitrogen will increase crop
yield. It may also
be useful to determine the value of a phosphorus
or potassium fertilizer on each of the important crops in
the system.
Second, the nature of the soil or soils in the region should
be
identified. Factors
to consider here would be the depth, texture
(soil particle size), structure (crumbs, blocks, plates),
organic
matter content, drainage, slope, and nutrient content of the
soil, including the acidity or alkalinity (pH).
The third factor to consider is the suitability of the crop
or
crops to the local soils, rainfall, temperature, length of
growing
season, ease of production, and marketability.
The proper
arrangement of crops on the farm and the best planting and
harvesting sequence also need to be assessed.
The final factor to be considered is the availability of
sources
of plant nutrients.
Are local deposits of nutrient-rich materials
available? If the pH
needs to be modified, is ground
limestone available locally? If organic matter is needed,
are
good sources available?
How could animal husbandry be better
utilized to furnish humus and nutrients to the soil?
If resources are not available locally, then nutrients may
need
to be imported into the region.
The organization of such supply
systems may be carried out by private businesses, the
government,
or community cooperatives.
Again, careful assessment and management
is necessary to make certain such resources are both
appropriate
and economically justified.
ADDITIONAL CONSIDERATIONS
Rainfall and Irrigation
Many of the new high-yielding crop varieties require large
amounts
of water and irrigation is often essential to increase
yield. This may
require great expense if water must be pumped
from a well or river.
Many agricultural development schemes have
run into considerable difficulties as water supplies became
depleted
or fuel costs increased sharply.
An additional consideration
is the expense of leveling the land to allow efficient
surface irrigation.
Also, for some soils, farmers need to prevent
the buildup of sodium and other salts caused by the
evaporation
of water after several years of surface irrigation.
Soil Texture and Drainage
Soil texture, which is the percentage of sand, silt, and
clay
particles in the soil, must be considered in the management
of
soil fertility. A
sandy soil (coarse texture) will not hold
fertilizer nutrients against leaching.
Therefore, fertilizer
should be added in small amounts and fairly frequently.
However,
such a loose soil is well drained and thus permits good
aeration
of both plant roots and soil organisms.
Organic matter (humus)
added to a sandy soil may increase the humus content and
also the
nutrient-holding capacity.
Many tropical sandy soils will not
hold humus for very long because of the extremely high rate
of
organic matter decomposition.
For such soils, the amount of clay
minerals is crucial since these tiny clay particles will
hold
most fertilizer nutrients by adsorption (physical and
chemical
attraction).
Silt particles, intermediate between sand and clay in size,
are
also intermediate in fertilizer-holding capacity.
Soils with a
high clay content may be tight and poorly drained, thus
decreasing
the oxygen availability to roots.
The addition of organic
matter to such soil will often greatly improve the crumb
structure
of the soil, permitting better water drainage and an
increased
supply of oxygen.
Unless a soil is well-drained, addition
of fertilizer will have little value in yield improvement.
Soil Reaction
Soil reaction refers to the hydrogen ion content of the
soil,
which can be measure using the pH scale.
A pH of below 6.5 is
considered an acid soil and is unsuitable for many
crops. The
addition of lime or limestone (calcium carbonate) will help
replace the hydrogen ions on the soil particles with
calcium,
raising the pH to a desirable level.
Again, the higher the clay
content or organic matter in the soil, the more calcium is
required
to replace the hydrogen on the clay or humus particles.
Some old soils that have been leached for centuries are
highly
acid and may require considerable treatment to make them
suitable
for certain crops.
Such soils may be suited to what are called
acid-loving crops (such as bermuda grass, cotton, cowpea,
peanut,
pineapple, sweet potato, coffee, and orchids).
Previous Experience and Available Plant Varieties
The importance of research experience cannot be
overemphasized in
considering the soil fertility system.
Such experience is
difficult to obtain because demonstrations and experiments
in
which just one variable at a time is being examined are hard
to
design, but there is no better way to determine plant
fertility
needs. When new
varieties of plants are being considered for use
in the cropping system, their response to soil fertility
must be
examined under each type of field condition.
Such research should
be done at an agricultural research center, if possible.
V. FUTURE DEVELOPMENT OF FERTILIZATION SYSTEMS
RESEARCH
New methods of supplying nutrients to plants are emerging.
Particularly
promising is the genetic modification of plants other
than legumes to accept nitrogen-fixing bacteria into nodules
on
their roots. With
the advent of this technology, a major milestone
in plant nutrition will have been reached.
Currently,
however, this type of genetic engineering is proving to be
more
complex than first anticipated.
Continued research in genetic engineering may produce
additional
genetic potential in crop plant growth and yield.
The revolutionary
type of plant breeding using tissue culture and haploidy
should make possible new genetic advances whose nature is
still
unknown. Tissue
culture takes single cells from a plant and grows
them into new plants.
If these single cells come from tissue
with one set of chromosomes (haploid), such as the cells
that
give rise to pollen grains, then the hidden or recessive
genetic
traits will appear.
This helps plant breeders deal with one gene
at a time.
Research on the interactions of plants in mixed culture
(growing
more than one crop in a field at a time) is still only in
the
beginning stages, mainly because the industrialized,
monoculture
type of cropping patterns have tended to overshadow the more
labor-intensive mixed culture technology.
Mixed culture requires
more harvesting and hand weeding since machines cannot
distinguish among the plants.
As certain regions of the world
concentrate more on multiple cropping (growing more than one
crop
together), the symbiotic effects of such systems will become
better known.
Symbiosis occurs when both crops benefit by being
grown together. One
crop may help the other (e.g., corn can
support climbing beans), while in return the second crop may
furnish nutrients to the first (beans fix nitrogen, which
the
corn may use).
ECONOMICS
The economics of food production in the future is a major
puzzle
for many persons attempting to forecast agricultural
trends. The
cost of industrially-based resources, so essential for much
"modern"
agriculture, is escalating rapidly.
Many North American
farmers find their labor-efficient products to be priced
above
the amount hungry nations can afford to pay.
For this reason,
the poorer countries are often advised to develop a national
food
policy of self-sufficiency, based on local soil fertility
resources.
The population pressure in most nations of the world is a
major
threat to many agricultural systems, especially those
requiring
fallow and crop rotation (different crops in different
seasons on
the same field). In
countries with land reform programs where
landless peasants are becoming landowners, the problem of
decreased
production for export often follows.
Economic pressures
on the nation for increased export earnings often are felt
by the
new landowners in the form of federal decrees.
For example, a
national government may require farmers to grow export crops
like
coffee or bananas, rather than food crops for local use;
often
farmers will resent these decrees.
Economic factors often frustrate
such programs because the new farmers are unable to produce
the export crop successfully.
As a result, the land returns to
the creditors and landlessness is again established.
There is a constant struggle for farmers to care for their
land
and their families while at the same time trying to adjust
to
international economic realities beyond their control.
The maintenance
and improvement of soil fertility is basic to farmers,
economic survival.
However, there is no guarantee of success
because factors beyond individual control may render all
efforts
futile. In the
last-analysis, the protection of soil fertility
and the economic viability of the agricultural sector must
be
part of the food policy of every national government.
BIBLIOGRAPHY/SUGGESTED READING LIST
Brady, Nyle. The
Nature and Properties of Soil. New York, New
York: MacMillan
and Sons Publishing Company, 1984.
Donahue, Roy L., Miller, Raymond W., and Shicklum, John C.
Soils,
An Introduction
to Soils and plant Growth. 5th edition.
Englewood
Cliffs, New Jersey: Prentice-Hall, Inc., 1983.
The Fertilizer Institute. The Fertilizer Handbook.
Washington,
D.C.: The
Fertilizer Institute, 1982.
Follett, Roy H., Murphy, Larry S., and Donahue, Roy L.
Fertilizers
and Soil
Amendments. Englewood Cliffs, New Jersey:
Prentice-Hall,
Inc., 1981.
McCune, Donald L. Fertilizers for Tropical and Subtropical
Agriculture.
Muscle Shoals,
Alabama: International Fertilizer
(undated).
Olson, R.A. Fertilizer Technology and Use. Washington, D.C.:
Soil
Science Society of America, 1971.
United Nations.
Fertilizers and Their Use. New
York, New York:
United Nations,
1978.
========================================
========================================

